ISMPO Insights
ESMO 2020: Recent updates and insights
Dr. Sneha Bothra Jain1, Dr. Sandeep Tiwari2
1MD, MRCPI, DNB (Medical Oncology), ECMO, Department of Medical Oncology Action Cancer Hospital, New Delhi
2DNB (Radiotherapy), DNB trainee (Medical Oncology) Department of Medical Oncology Action Cancer Hospital, New Delhi
Email : snehabothrajain@gmail.com
The year 2020 has had an unimaginable turn of events from the world going into a lockdown in view of a pandemic, to everything being virtual, including scientific meetings. The COVID 19 pandemic did not hold back the oncology community from continuing research and conducting scientific meetings to unfold the recent advances. European Society of Medical Oncology (ESMO) 2020 was also one of the remarkable meeting held in virtual format with personal experience and vast participation oncologists across the globe.
The field of oncology is rapidly evolving, and we are currently entering in an era of personalized medicine which consists of targeted therapy and immunotherapy, minimizing collateral damage of normal tissue while treating malignant cells more precisely. We present the major updates of ESMO 2020 meeting. This meeting did update on a few unmet needs that we are facing in oncology.
BREAST CANCER
IMpassion031[1]
Harbeck et al., presented this randomized phase III trial of neoadjuvant systemic therapy (NAST) where treatment naïve stage II/III triple negative breast cancer (TNBC) patients with tumor size > 2 cm were randomized in two groups. In Group A (n=168)- nanoparticle albumin-bound (Nab)-paclitaxel (12 weeks) Adriamycin + cyclophosphamide (AC) for eight weeks followed by surgery followed by observation (Control group) versus group B (n=165) - Nab paclitaxel + atezolizumab (12 week) Atezolizumab + (AC) for eight weeks duration followed by surgery followed by 11 doses of atezolizumab. They were stratified on basis of disease stage (stage II versus III), PD- L1 status (≥ 1% versus < 1%). The primary endpoints were pathological Complete response (pCR) in intention to treat (ITT) population and PD-L1 subpopulation and secondary endpoint were event free survival (EFS), disease free survival (DFS), overall survival (OS) in all patients and PD-L1 subpopulation.
In the ITT analysis, pCR was achieved in 41% of the control group versus (vs) 58% of the atezolizumab group (p=0.0044). In the PD-L1–positive population, pCR was achieved in 69% vs 49% group B and group A, respectively. There were no new safety signals and most of the adverse events (AEs) were related to the chemotherapy backbone. Investigators suggested that this combination may offer an improvement in pCR with neoadjuvant therapy in TNBC patients.
Insight: NAST is very important in TNBC and improving the pCR rates with NAST has been the goal with this aggressive breast cancer. However, not many drugs have been able to improve this in TNBC. Immunotherapy in this setting with data on improvement in PFS looks promising and gives a ray of hope for TNBC patients.
monarchE [2]
Johnston et al., presented this phase III open label trial where HR+/HER2- early breast cancer patients with high risk of recurrence. This included patients with ≥ 4 positive axillary nodes, or 1-3 positive nodes and at least one of the following: tumor size ≥ 5 cm, histological grade-3, Ki67 index ≥ 20% and completed primary treatment were randomized in to two groups; Group-A had CDK 4/6 inhibitor (Abemaciclib 150 mg BID) up to two years + standard adjuvant endocrine therapy (ET) for 5-10 years vs standard adjuvant ET (group - B). The primary endpoint was invasive disease-free survival (IDFS) and secondary endpoints were distant recurrence free survival (DRFS), OS, patient reported outcomes (PROs), safety and pharmacokinetics (PK).
A total of 5637 patients were randomized 1:1 in both arms. Patients had significantly improved IDFS, DRFS in abemaciclib as compared to standard ET. At 24 months, IDFS was 92.2% vs 88.7% (p=0.01) and DRFS 93.6% vs 90.6% (p=0.01) in the investigational arm and standard arm, respectively. Safety profile similar with the known adverse effects of abemaciclib. This was the first trial with a CDK 4/6 inhibitor (abemaciclib) to demonstrate significant benefit in combination with standard adjuvant ET in HR + and HER2 negative early breast cancer (EBC) population although more mature data is awaited regarding the same.
Insight: Therapy for patients with hormone positive breast cancer with high risk of recurrence is an unmet need, as any recurrence adds to further burden of treatment, hospital visits and financial implications. This is more so for the Indian population, where the disease at presentation is more advanced and we need better strategies for prevention of relapse. Addition of abemaciclib has shown a PFS advantage and this gives some optimism to the high-risk group. More mature data is awaited for the same. In a similar trial setting, where all stage II/III hormone positive patients were included for adjuvant palbociclib in addition to the standard endocrine therapy, it showed no benefit of addition of CDK4/6 inhibitor to the standard adjuvant therapy. So, to have the best outcomes, we need a good selection of patients.
ASCENT - Sacituzumab Govitecan (IMMU-132) [3, 4]
Khoury et. al. [3] described that there is a novel molecule identified trophoblast antigen 2 (TROP-2, which is a glycoprotein first described as a surface marker of trophoblast cells) but is also increased in many solid cancers (e.g., Breast) with lower expression in certain normal tissues. It regulates cancer growth, invasion and spread by several signaling pathways. When active forms of irinotecan SN-38 were attached to anti TROP 2 immunoglobulin, it formed antibody drug conjugate (ADC), thereby increasing solubility and selectively increasing drug delivery to tumor cells.
Bardia et al., [4] presented this randomized Phase III trial (ASCENT) which included patients with metastatic TNBC (mTNBC) who received ≥ 2 prior lines of chemotherapy, progression within 12 months of (neo)adjuvant therapy, ECOG PS 0/1, measurable disease on basis of RECIST v1.1 and were stratified on basis of geography, no prior chemotherapy (2-3 vs >3), brain metastasis (yes vs no). A total of 529 patients were randomized 1:1 into two groups - Group - A received Trop-2 antibody sacituzumab govitecan 10 mg/kg on Day 1, 8 on 21-day cycle, vs Group B single agent chemotherapy (Eribulin or vinorelbine or gemcitabine, or capecitabine) treated until disease progression or intolerable toxicity. Primary endpoint was PFS by independent review committee (in patients with no brain metastasis). Secondary end points included were PFS, OS, Overall response rate (ORR), duration of response (DOR), time to response (TTR) and safety of sacituzumab govitecan.
The trial met its primary and secondary endpoints where there was significant improvement of median PFS of 5.6 vs 1.7 months (HR: 0.41; P < .0001), median OS of 12.1 vs 6.7 months (HR: 0.48; P < .0001) and ORR of 35% vs 5% in the sacituzumab govitecan and control groups, respectively. Sacituzumab govitecan was well tolerated. Investigators concluded that sacituzumab govitecan should be considered a new standard of care mTNBC with prior lines of therapy. There were no treatment-related deaths, severe cardiovascular toxicity, grade ≥ 3 neuropathy, or grade ≥ 4 ILD with sacituzumab govitecan.
Insight: This trial tested a novel molecule in heavily pretreated metastatic TNBC patients against the standard of care giving a potential option in this subset of patients. Although how useful it gets to the general population in India, depends on the launch in our country and its pricing, as these newer drugs are having heavy financial implications.
GENITOURINARY CANCERS
PROSTATE
STAMPEDE [5] : Long-Term Results of Androgen Deprivation Therapy (ADT) + abiraterone acetate in hormone-naïve or sensitive M1 Hormone Sensitive Prostate Cancer (HSPC)
In 2017, LATITUDE trial and STAMPEDE trial, ARM-G demonstrated improvement of OS who received abiraterone acetate + prednisolone (AAP) and ADT vs ADT alone in metastatic prostate cancer. After 3 years, James et al. [5] presented long term results of STAMPEDE trial arm G at ESMO 2020. A total of 1917 patients were randomized 1:1 to ADT alone or ADT + AAP. Primary endpoint was OS and secondary end point was failure free survival (FFS), PFS, metastatic free survival (MFS), skeletal related event (SREs) and toxicity.
Long term results of the trial were suggestive of significant OS benefit, with median OS of 6.6 years with AAP + ADT versus 3.8 years with ADT alone (p=0.0000000003) and significant difference in OS in low risk, high risk patient, low volume and high-volume disease. There was significant benefit in median FFS of 4.3 years vs 0.96 year (p=0.0000000001) and median PFS of 5.9 vs 3.2 years in AAP + ADT and ADT alone, respectively. There was significant reduction in SREs observed with AAP + ADT vs ADT alone. Safety profile with abiraterone acetate + prednisone was similar to the previous trials.
Insight: Durable long-term response in terms of OS, PFS, FFS with abiraterone + prednisone + ADT in low risk, high risk, low volume and high volume metastatic prostate cancer. These long-term data support the addition of abiraterone + prednisone to ADT as preferred treatment for hormone-naïve or sensitive M1 CSPC
UROTHELIAL CANCER
KEYNOTE-361 was a phase III randomized trial and was presented by Alva et al. [6] at the ESMO 2020. In this trial, 1010 patients with treatment naïve metastatic or advanced urothelial carcinoma with good performance status were randomized into three arms: Arm A (n=352)- six cycle gemcitabine + cisplatin or carboplatin every three weekly (CT alone) versus Arm-B (n=351) (Arm A + injection pembrolizumab) (Pembrolizumab + CT) followed by three weekly pembrolizumab for 29 cycles versus Arm-C (n=307) pembrolizumab alone for 35 cycles. Patients were stratified on the basis of platinum-based chemotherapy and percentage of PDL-1 positivity (<10% vs > 10%). Primary endpoint was PFS (Blinded independent central review - BICR) and OS with secondary endpoints being ORR, disease control rate (DCR), DOR and safety. In this BICR, PFS and OS were not significantly improved with the addition to pembrolizumab to CT. however, median PFS reported by investigator was 8.3 months with pembrolizumab + CT vs 7.1 months with CT; [HR: 0.78 (95% CI: 0.65-0.93), p= .0033] and median OS 17 months with pembrolizumab + CT vs 14.3 months [HR: 0.86 (95% CI: 0.72-1.02), P = .0407]. Median OS in CT vs pembrolizumab alone was 15.6 vs 14.3 months, respectively 16.1 vs 15.2 months in patients with PDL1 >10%, and was not significant statistically.
DANUBE trial: It is a Phase III randomized trial presented by Powles et al., [7] at ESMO 2020. In this trial, a total of 1032 adult patients with treatment naive, unresectable, locally advanced or metastatic urothelial cancer, ECOG PS ≤ 1, CrCl ≥ 30 mL/min, measurable disease size ≥ 1 were randomized into three arms; Arm-A (n=344) standard chemotherapy (six cycle Gemcitabine + cisplatin/carboplatin), Arm-B (n=346) - inj durvalumab administered every 4 weeks until disease progression and Arm-C(n=342) inj Durvalumab (till disease progression)+ inj Tremelimumab (four cycles) in four weekly interval. They were stratified by platinum eligibility, PDL1 High or low.
Primary endpoints were OS with durvalumab vs CT in PD-L1 high patients, OS with durvalumab + tremelimumab vs CT in intention to treat ITT population while secondary endpoints were OS with durvalumab vs CT in ITT population, OS with durvalumab + tremelimumab vs CT in PD-L1 high patients, PFS, ORR and DOR. The trial failed to meet its primary or major secondary endpoints. Median OS with durvalumab vs CT in ITT population was 13.2 months vs 12.1 months [HR: 0.99 (0.83-1.17)], while in durvalumab + tremelimumab vs CT, HR was 0.85 (95% CI: 0.72-1.02), p = .075. Median OS with durvalumab + tremelimumab vs CT in PD-L1 high patients: 17.9 vs 12.1 months; HR: 0.74 (0.59-0.93), while in durvalumab vs chemotherapy in PDL-1- high population 0.80 (95% CI: 0.71- 1.11).
Insight: The present evidence suggests that there is no OS benefit to either combination of anti PD-1+ chemotherapy or anti-PD-L1+ CTLA-4 versus chemotherapy alone. Higher ORR with CT alone than durvalumab even in the PD-L1–high population. In the context of the above two trials, first line preferred treatment option will remain platinum based chemotherapy and checkpoint inhibitors should be reserved for subsequent therapy or salvage therapy as second line therapy.
RENAL CELL CARCINOMA (RCC)
CheckMate 9ER [8]
In Advanced or Metastatic Renal cell carcinoma at present first line treatment in favorable risk patients is pembrolizumab with axitinib or sunitinib or pazopanib while in intermediate and poor risk patients, it is nivolumab + ipilimumab.
Checkmate 9ER by Choueiri et al. [8] presented the role of cabozantinib + nivolumab in treatment naïve locally advanced or metastatic clear cell renal cell carcinoma. Patient were stratified by International Metastatic RCC Database Consortium (IMDC) risk score, PDL1 expression, bone metastasis and randomized into two arms; Arm-A (n=328) had tab sunitinib 50 mg once a day for four weeks on and two weeks off versus Arm-B (n=323) tab cabozantinib 40 mg + inj nivolumab 240 mg every two weeks until disease progression or unacceptable toxicity.
At the first interim analysis with median follow up of 18.1 months, the median PFS was 16.6 months vs 8.3 months for the combination arm and sunitinib, respectively [HR: 0.51 (95% CI, 0.41-0.64; P < .0001)]. Health related quality of life improved, risk of death reduced by 50% and objective response rate were increase by 29% in experimental group. Survival and response noted in all subgroups of patients.
Insight: With more and more tyrosine kinase inhibitors (TKI) showing improved outcomes, now there is wide range of options for first line management of metastatic RCC.
GASTROINTESTINAL CANCERS
CheckMate 577: Adjuvant Nivolumab vs Observation Following Neoadjuvant CRT and Resection in EC/GEJC:
At present there is no adjuvant treatment option for patients who did not achieve pCR in stage II/III esophageal and gastroesophageal junction (GEJ) cancer treated with neoadjuvant chemoradiation followed by surgical resection. Dr Kelly et al., [9] presented this randomized, double blind, phase III trial where patients of stage II/III esophageal and GEJ cancer treated with neoadjuvant chemoradiation followed by surgical resection and not achieving pCR were stratified on basis of histology (adenocarcinoma vs squamous cell carcinoma), pathologic lymph node status (≥ ypN1 vs ypN0) and tumor cell PD-L1 expression (≥ 1% vs < 1%). Patients were randomized into two groups; inj nivolumab 240 mg (Q2W) × 16 weeks, then 480 mg Q4W up to one year or disease progression or intolerable due to side effect vs placebo group. Primary end point was DFS and secondary endpoints were OS and OS rate at one, two and five years.
At six months, DFS was 72% in nivolumab and 63% in the placebo group. At a median follow up of 24.4 months, median DFS doubled in nivolumab arm (22.4 months) vs placebo arm (11.0 months) [HR: 0.69 (96.4% CI: 0.56-0.86; P = .0003)]. There was 31% reduction in risk of recurrence and death. Investigators concluded that nivolumab adjuvant therapy provided a statistically significant and clinically meaningful DFS improvement vs placebo in this subset of patients. DFS benefit was seen in all patients irrespective of PD-L1 expression and tumor PD-L1 level was not a good predictor of outcomes. Adjuvant nivolumab could become a new standard of care in patients with resected esophageal and gastroesophageal junction cancers.
Phase III CheckMate 649: First line Nivolumab + CT vs CT in Advanced Gastroesophageal Cancers
Mochler et al., [10] included patients with previously untreated, unresectable, advanced or metastatic gastric cancer, gastroesophageal junction or esophageal adenocarcinoma who were HER2 negative with ECOG PS 0-1 (regardless of PDL1 expression). Patients were stratified by PD-L1 (≥ 1% vs < 1%), region (Asia vs US/Canada vs rest of world), ECOG PS (0 vs 1), CT backbone (XELOX vs FOLFOX). Patients were randomized into three group; Group A - XELOX Q3W or FOLFOX Q2W; Group B - Nivolumab 360 mg + XELOX Q3W or nivolumab 240 mg + FOLFOX Q2W and Group C - Nivolumab + ipilimumab Q3W x 4 followed by nivolumab 240 mg Q2W. Treatment was given until disease progression (treatment beyond PD permitted for nivolumab + CT) or unacceptable toxicity. Co-primary endpoints were OS and PFS in patients with PD-L1 CPS ≥ 5 and secondary endpoints were ORR, OS and PFS in all randomized patients and with PD-L1 CPS ≥ 10 and ≥ 1.
There was significant improvement in survival outcomes in all patients that were randomized. In patients with PD-L1 CPS ≥ 5 (coprimary endpoints), the median OS was 14.4 vs 11.1 months (HR: 0.71; P < .0001) and median PFS was 7.7 vs 6.1 months (HR: 0.68; P < .0001) in nivolumab + CT versus CT alone, respectively. In all patients randomized, the median OS was 13.8 months versus 11.6 months (HR 0.80, p=0.0002) in nivolumab + CT versus CT alone, respectively. Investigators concluded that first-line treatment with nivolumab + CT may be new potential standard of care for patients with advanced gastroesophageal cancers.
ATTRACTION-4 [11] : First line Nivolumab + Chemotherapy vs Chemotherapy Alone for Advanced Gastric/GEJ Cancer
[9]After improvement of survival with nivolumab in patients with metastatic esophageal cancer, it was attempted to be used in the front line setting with chemotherapy in the ATTRACTION-4 trial. It was a randomized double-blind phase 2/3 trial of nivolumab + chemotherapy versus chemotherapy in Asian patients with metastatic esophageal cancer. A total of 724 patients were included in the study and were randomized 1:1 to receive nivolumab + chemotherapy (n=362) or placebo + chemotherapy (n=362) administered once in every three weeks. Chemotherapy backbone used was oxaliplatin + S-1 or capecitabine. The co-primary end points were median PFS and OS (centrally assessed).
The interim analysis was done as median follow-up of 11.6 months. The median PFS was 10.5 versus 8.3 months (HR 0.68; 98.51% CI 0.51-0.90; p=0.0007) in the chemotherapy + nivolumab versus chemotherapy arm alone. At the final analysis with median follow up of 26.6 months, although the PFS was improved in the nivolumab arm, median OS was 17.5 months versus 17.2 months, in the, respectively, showing no significant improvement in the OS. The ORR was higher in nivolumab + CT arm (57.5%) versus placebo+ CT (47.5%) (p=0.0088).
KEYNOTE-590: First line Pembrolizumab + CT vs Placebo + CT in Patients with Advanced Esophageal Cancer
In this randomized phase-3 trial, Kato et al., [12] attempted to identify the role of pembrolizumab in advanced esophageal cancer in first line setting. They stratified patients on basis of region, disease and performance status. They included treatment naïve unresectable locally advanced or metastatic esophageal cancer or advance or metastatic GEJ Siewert type-1 adenocarcinoma. These patients were then randomized into two groups; Group - A Pembrolizumab 200 mg IV Q3W for ≤ 35 cycles + chemotherapy versus Placebo IV Q3W for ≤ 35 cycles + chemotherapy. The chemotherapy backbone used was 5-FU 800 mg/m2 on Days 1-5 Q3W for ≤ 35 cycles + cisplatin 80 mg/m2 IV Q3W for ≤ 6 cycles. Coprimary endpoints were OS and PFS and secondary endpoint was ORR.
Patients of advanced esophageal cancer (n=749) were randomized 1:1 to pembrolizumab + chemotherapy (n=373) and placebo + chemotherapy (376). At median follow up of 10.8 months, the following results were presented:
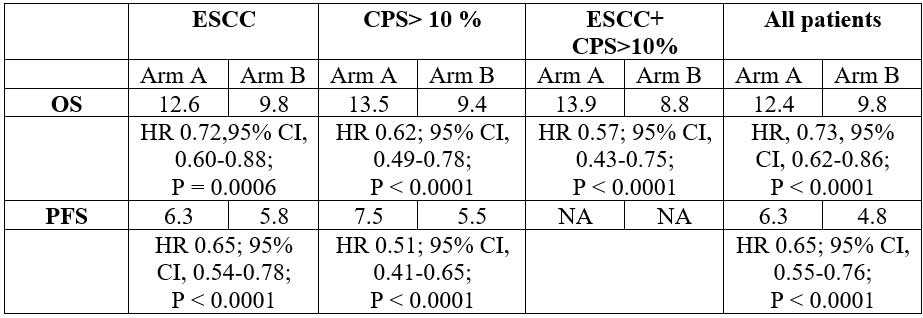
OS and PFS favored pembrolizumab + CT vs CT in most patient subgroups. Investigators concluded that first-line pembrolizumab + CT represents new standard of care for patients with locally advanced/metastatic esophageal cancer.
Insight: CheckMate 649 and KEYNOTE-590: This is a historic year for esophageal and GEJ both. CheckMate 649 and KEYNOTE-590 showed improvement in OS with the addition of anti–PD-1 therapies to CT in the first-line setting. Key differences between the studies include patient population and detection of PD-L1 expression, high percentage of squamous cell carcinoma (SCC) in KEYNOTE-590 with no gastric tumors), in CheckMate 649 included patients with gastric, esophageal, and GEJ adenocarcinomas.
LUNG CANCER
Phase III ADAURA: Adjuvant Osimertinib vs Placebo in Completely Resected EGFR-Mutated NSCLC
Tsuboal et al., [13] presented a phase-3 trial in patients with primary non-squamous NSCLC with EGFR ex19del or L858R and completely resected stage IB/II/IIIA NSCLC with negative margins with age ≥ 18 years (≥ 20 years in Japan/Taiwan) with WHO PS 0/1 and brain imaging done. Adjuvant CT was permitted and maximum time from surgery to randomization was ten weeks without adjuvant CT and 26 weeks with adjuvant CT. They were stratified on basis of stage (IB vs II vs IIIA), EGFR mutation (exon 19 deletion vs L858R) and race (Asian vs Non-Asian).
A total of 682 patients were randomized into two groups, group A (n=339) was tablet osimertinib 80 mg daily and group B (n=343) was placebo until three year or recurrence. Early unblinding was done due to efficacy and at time of unblinding, the trial had completed enrollment and all patients had more than one year of follow-up. Primary endpoint was investigator-assessed DFS in patients with stage II/ IIIA disease and secondary endpoints were DFS, OS and safety in overall population. The landmark analysis DFS rates were at years two to five.
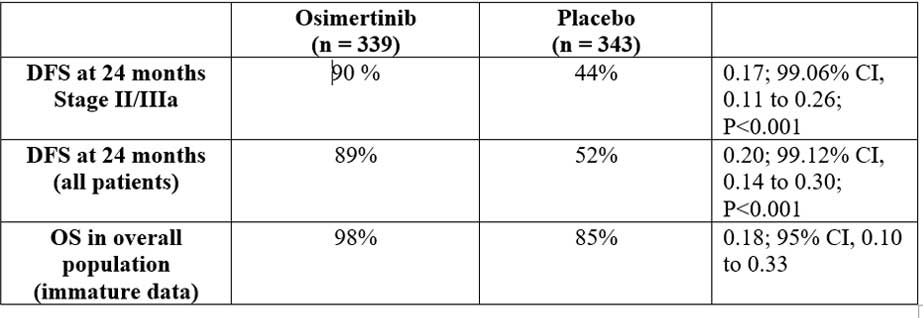
Insight: Adjuvant therapy has been an unmet need in completely resected NSCLC, and addition of EGFR TKI in sensitizing EGFR mutant NSCLC with significant improvement of two-year DFS, brings in a new hope for these patients. The most important is to look for the overall survival outcomes for these patients as compared to the chemotherapy arm, giving rise to the actual advantage of administering these drugs in the adjuvant setting
CodeBreaK 100: Sotorasib in KRASG12C-Positive NSCLC
Phase I/II trial presented by Hong et al., [14] included adult patients with locally advanced or metastatic NSCLC with KRASp.G12C mutation with prior standard therapy, ECOG PS 0-2 with no active brain metastasis and measurable disease by RECIST v1.1. They were given tab sotorasib in different dose schedule (phase I) in different cohort of patient 180 mg, 360mg, 720mg, 960 mg respectively followed by phase II (dose expansion) trial. The primary endpoints were safety and secondary endpoints were pharmacokinetics, ORR, DOR, DCR, PFS, duration of SD.
Sotorasib demonstrated a favorable safety profile in patients with locally advanced or metastatic NSCLC with the KRASp.G12C mutation, no dose-limiting toxicities or treatment-related fatal adverse events were seen. Phase II dose was identified as 960-mg. Durable disease control observed in this heavily pretreated patient population with a confirmed ORR of 35.3%, DCR of 91.2% in the 960 mg cohort. Investigators concluded that further study is warranted of sotorasib as monotherapy or in combination regimens in patients with solid tumors with KRASp.G12C mutation.
Insight: Although this data appears promising for RAS mutant tumors, a phase III trial for these subgroup of NSCLC, CodeBreaK 200, is currently enrolling (NCT04303780), and the results of this trial will be eagerly awaited.
CheckMate 9LA: Patient-Reported Outcomes with first-line Nivolumab + Ipilimumab + CT vs CT in NSCLC
A phase III trial was presented by Rack et al. [15] which included patients with stage IV or recurrent NSCLC with no previous systemic treatment having no sensitizing EGFR/ALK alteration, ECOG PS 0/1. They were stratified by PD-L1 expression (≥ 1% vs < 1%), sex, and histology (squamous vs non-squamous) randomized into two groups, Group A - (n=361) Nivolumab 360 mg Q3W + Ipilimumab 1 mg/kg Q6W+ chemotherapy Q3W two cycles versus chemotherapy alone (n=358) q3w (4 cycles) until PD, unacceptable toxicity, or for two years for immunotherapy. Primary endpoint was OS and secondary endpoints were PFS, ORR, efficacy by tumor PD-L1 expression.
After a median follow-up of 13·2 months, median OS was 15·6 months versus 10·9 months (9·5–12·6) in the experimental group and control group, respectively (HR 0·66 [95% CI 0·55–0·80]). Serious treatment-related AEs of any grade occurred in 106 (30%) patients in the experimental group and 62 (18%) in the control group. The nivolumab + ipilimumab + two chemotherapy groups improved or maintained symptom burden and overall health status as compared with four cycles of chemotherapy.
Insight: The main aim of this trial was to see whether reducing the number of chemotherapy cycles to immunotherapy reduces the short-term morbidity without compromising on the efficacy. Although there was improvement in the survival outcomes, the main discussion remains about the accessibility of these drugs to the population at large.
References
- 1. Mittendorf EA. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial - PubMed [Internet]. [cited 2021 Jan 28]. Available from: https://pubmed.ncbi.nlm.nih.gov/32966830/
- 2. Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol Off J Am Soc Clin Oncol. 2020 Dec 1;38(34):3987–98.
- 3.Khoury K, Feldman R, Pohlmann P, Heeke A, Gatalica Z, Veloso Y, et al. Molecular characterization of trophoblast cell surface antigen 2 (Trop-2) positive triple negative breast cancer (TNBC). J Clin Oncol. 2019 May 20;37:e14651–e14651.
- 4. Bardia A, Tolaney SM, Loirat D, Punie K, Oliveira M, Rugo HS, et al. LBA17 ASCENT: A randomized phase III study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with previously treated metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2020 Sep 1;31:S1149–50.
- 5. James N, Rush H, Clarke N, Attard G, Cook A, Dearnaley D, et al. 611O Abiraterone acetate plus prednisolone for hormone-naïve prostate cancer (PCa): Long-term results from metastatic (M1) patients in the STAMPEDE randomised trial (NCT00268476). Ann Oncol. 2020 Sep 1;31:S509.
- 6. Alva A. LBA23 Pembrolizumab (P) combined with chemotherapy (C) vs C alone as first-line (1L) therapy for advanced urothelial carcinoma (UC): KEYNOTE-361 - Annals of Oncology [Internet]. [cited 2021 Jan 30]. Available from: https://www.annalsofoncology.org/article/
- 7. Powles T. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial - The Lancet Oncology [Internet]. [cited 2021 Jan 30]. Available from: https://www.thelancet.com/journals/lanonc/
- 8. Choueiri TK. 696O_PR Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial - Annals of Oncology [Internet]. [cited 2021 Jan 30]. Available from: https://www.annalsofoncology.org/article
- 9. ESMO Virtual Congress 2020. Adjuvant Nivolumab Offers Novel Treatment Advance in Esophageal/Gastroesophageal Junction Cancer [Internet]. PracticeUpdate. [cited 2021 Jan 30]. Available from: https://www.practiceupdate.com/content/esmo-2020
- 10. Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, et al. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol. 2020 Sep 1;31:S1191.
- 11. ESMO Virtual Congress 2020. ATTRACTION-4: First-line Nivolumab + Chemotherapy vs Chemotherapy Alone for Advanced Gastric/GEJ Cancer - Google Search [Internet]. [cited 2021 Jan 30]. Available from: https://www.google.com/Advanced+Gastric
- 12. ESMO Virtual Congress 2020. Immunotherapy is Beneficial in Gastric and Oesophageal Cancers, Studies Show [ESMO2020 Press Release] [Internet]. [cited 2021 Jan 30]. Available from: https://www.esmo.org/newsroom
- 13. Wu Y-L, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 2020 Oct 29;383(18):1711–23.
- 14. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. New England Journal of Medicine. 2020 Sep 24;383(13):1207-17.
- 15. Reck M, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. LBA59 First-line nivolumab (NIVO) + ipilimumab (IPI) combined with 2 cycles of platinum-based chemotherapy (chemo) vs 4 cycles of chemo in advanced non-small cell lung cancer (NSCLC): Patient-reported outcomes (PROs) from CheckMate 9LA. Ann Oncol. 2020 Sep;31:S1187–8.
Courtesy - Indian Journal of Medical and Paediatric Oncology (IJMPO)
Editor-in-Chief - Dr. Padmaj Kulkarni